Minizide
Generic name: prazosin hydrochloride and polythiazide
Dosage form: capsules
Drug class:Antiadrenergic agents (peripheral) with thiazides
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
FOR ORAL ADMINISTRATION
On This Page
This fixed combination drug is not indicated for initial therapy of hypertension. Hypertension requires therapy titrated to the individual patient. If the fixed combination represents the dose so determined, its use may be more convenient in patient management. The treatment of hypertension is not static, but must be re-evaluated as conditions in each patient warrant.
Minizide Description
Minizide® is a combination of MINIPRESS® (prazosin hydrochloride) plus RENESE® (polythiazide).
MINIPRESS (prazosin hydrochloride), a quinazoline derivative, is the first of that chemical class of antihypertensives. It is the hydrochloride salt of 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furoyl) piperazine and its structural formula is:
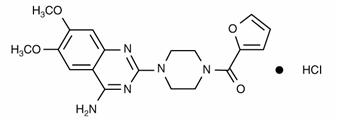
It is a white, crystalline substance, slightly soluble in water and isotonic saline, and has a molecular weight of 419.87. Each 1 mg capsule of MINIPRESS (prazosin hydrochloride) contains drug equivalent to 1 mg free base.
RENESE (polythiazide) is an orally effective, non-mercurial diuretic, saluretic, and antihypertensive agent.
It is designated chemically as 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-2-methyl-3-[[(2,2,2-trifluoroethyl)thio]methyl]-,1,1-dioxide, and has the following structural formula:
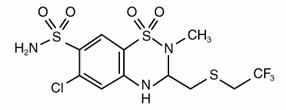
It is a white, crystalline substance insoluble in water, but readily soluble in alkaline solution.
Inert ingredients in the formulations are: hard gelatin capsules (which may contain Blue 1, Green 3, Red 3 and other inert ingredients); magnesium stearate; sodium lauryl sulfate; starch; sucrose.
Minizide - Clinical Pharmacology
Minizide (prazosin hydrochloride/polythiazide)
Minizide produces a more pronounced antihypertensive response than occurs aft...



