Minocin Injection
Generic name:minocycline hydrochloride
Dosage form: injection
Drug class:Tetracyclines
Medically reviewed by Drugs.com. Last updated on Jul 1, 2021.
Rx Only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of MINOCIN® (minocycline) for Injection and other antibacterial drugs, MINOCIN® (minocycline) for injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
On This Page
Minocin Injection Description
MINOCIN (minocycline) for Injection, is a sterile formulation of a semisynthetic derivative of tetracycline. The chemical name of minocycline is 4,7-Bis(dimethylamino)- 1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a- tetrahydroxy-1,11-dioxo-2- naphthacenecarboxamide monohydrochloride.
Its structural formula is:
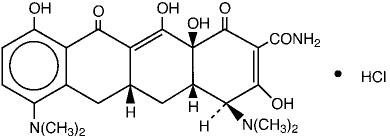 | ||
| C23H27N3O7∙HCl | M.W. 493.94 | |
MINOCIN is supplied as a sterile yellow to amber lyophilized powder for intravenous infusion. Each vial contains minocycline HCl equivalent to 100 mg minocycline, 269 mg magnesium sulfate heptahydrate (2.2 mEq of magnesium) (an inactive ingredient) and sodium hydroxide (to adjust pH). When reconstituted with 5 mL of Sterile Water for Injection USP the pH ranges from 4.5 to 5.0.
Minocin Injection - Clinical Pharmacology
Following a single dose of Minocin 200 mg administered intravenously to 10 healthy male subjects, serum concentrations of minocycline ranged from 2.52 to 6.63 mcg/mL (average 4.18 mcg/mL) at the end of infusion and 0.82 to 2.64 mcg/mL (average 1.38 mcg/mL) after 12 hours. In a group of 5 healthy male subjects,...



