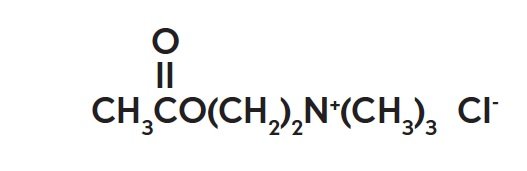Miochol-E
Generic name:acetylcholine chloride
Dosage form: intraocular solution
Drug class:Ophthalmic glaucoma agents
Medically reviewed by Drugs.com. Last updated on Jan 1, 2021.
On This Page
The Miochol-E brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
(acetylcholine chloride
intraocular solution)
20 mg/2 mL (10 mg/mL)
Sterile
1:100 with Electrolyte Diluent
FOR INTRAOCULAR USE ONLY
DESCRIPTION
Miochol™-E (acetylcholine chloride intraocular solution) is a parasympathomimetic preparation for intraocular use. It is packaged in a blister pack containing one vial and one diluent ampule. The vial contains 20 mg acetylcholine chloride and 56 mg mannitol. The accompanying ampule contains 2 mL of a modified diluent of calcium chloride dihydrate, magnesium chloride hexahydrate, potassium chloride, sodium acetate trihydrate, and sterile water for injection.
The reconstituted liquid will be a sterile isotonic solution (275–330 milliosmoles/kg) containing 20 mg acetylcholine chloride (1:100 solution) and 2.8% mannitol. The pH range is 5.0–8.2. Mannitol is used in the process of lyophilizing acetylcholine chloride, and is not considered an active ingredient.
The chemical name for acetylcholine chloride, C7H16ClNO2, is Ethanaminium, 2-(acetyloxy)-N,N,N-trimethyl-, chloride and is represented by the following chemical structure:
CLINICAL PHARMACOLOGY
Acetylcholine is a naturally occurring neurohormone which mediates nerve impulse transmission at all cholinergic sites involving somatic and autonomic nerves. After release from the nerve ending, acetylcholine is rapidly inactivated by the enzyme acetylcholinesterase by hydrolysis to acetic acid and choline.
Direct application of acetylcho..