Analpram Advanced
Generic name: hydrocortisone acetate and pramoxine hydrochloride
Dosage form: cream - topical kit
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
DESCRIPTION:
Analpram® HC Cream 2.5% is a topical preparation containing hydrocortisone acetate 2.5% w/w and pramoxine hydrochloride 1% w/w in a hydrophilic cream base containing stearic acid, cetyl alcohol, Aquaphor®, isopropyl palmitate, polyoxyl 40 stearate, propylene glycol, potassium sorbate, sorbic acid, triethanolamine lauryl sulfate, and purified water.
Topical corticosteroids are anti-inflammatory and anti-pruritic agents. The structural formula, chemical name, molecular formula and molecular weight for active ingredients are presented below.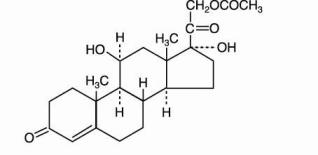
hydrocortisone acetate
Pregn-4-ene-3,20-dione, 21-(acetyloxy)-11, 17-dihydroxy-, (11-beta)-
C 23H 32O 6; mol. wt.: 404.50
pramoxine hydrochloride
4-(3-(p-butoxyphenoxy)propyl)morpholine hydrochloride
C 17H 27NO 3.HCl; mol. wt.: 329.87
CLINICAL PHARMACOLOGY:
Topical corticosteroids share anti-inflammatory, anti-pruritic and vasoconstrictive actions.
The mechanism of anti-inflammatory activity of topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Pramoxine hydrochloride is a topical anesthetic agent which provides temporary relief from itching and pain. It acts by stabilizing the neuronal membrane of nerve endings with which it comes into contact.
Pharmacokinetics:
The extent of percutaneous absorption of topical corticosteroids is determined by many factors



