Mycelex
Generic name:clotrimazole
Dosage form: Troche for Topical Oral Administration
Drug classes:Topical antifungals, Vaginal anti-infectives
Medically reviewed by Drugs.com. Last updated on Jul 22, 2021.
On This Page
The Mycelex brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Mycelex Description
Each Mycelex® Troche contains 10 mg clotrimazole [1-(o-chloro-α,α-diphenylbenzyl) imidazole], a synthetic antifungal agent, for topical use in the mouth.
Structural Formula:
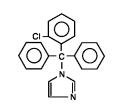
Chemical Formula:
C22H17CIN2
The troche dosage form is a large, slowly dissolving tablet (lozenge) containing 10 mg of clotrimazole dispersed in dextrose, microcrystalline cellulose, povidone, and magnesium stearate.
Mycelex - Clinical Pharmacology
Clotrimazole is a broad-spectrum antifungal agent that inhibits the growth of pathogenic yeasts by altering the permeability of cell membranes. The action of clotrimazole is fungistatic at concentrations of drug up to 20 mcg/mL and may be fungicidal in vitro against Candida albicans and other species of the genus Candida at higher concentrations. No single-step or multiple-step resistance to clotrimazole has developed during successive passages of Candida albicans in the laboratory; however, individual organism tolerance has been observed during successive passages in the laboratory. Such in vitro tolerance has resolved once the organism has been removed from the antifungal environment.
After oral administration of a 10 mg clotrimazole troche to healthy volunteers, concentrations sufficient to inhibit most species of Candida persist in saliva for up to three hours following the approximately 30 minutes needed for a troche to dissolve. The long term persistence of drug in saliva appears to be related to the slow release of clotrimazole from the oral mucosa to which the drug is apparently boun..



