Myleran
Generic name:busulfan
Dosage form: tablet, film coated
Drug class:Alkylating agents
Medically reviewed by Drugs.com. Last updated on May 1, 2021.
On This Page
Myleran is a potent drug. It should not be used unless a diagnosis of chronic myelogenous leukemia has been adequately established and the responsible physician is knowledgeable in assessing response to chemotherapy.
Myleran can induce severe bone marrow hypoplasia. Reduce or discontinue the dosage immediately at the first sign of any unusual depression of bone marrow function as reflected by an abnormal decrease in any of the formed elements of the blood. A bone marrow examination should be performed if the bone marrow status is uncertain.
SEE WARNINGS FOR INFORMATION REGARDING BUSULFAN-INDUCED LEUKEMOGENESIS IN HUMANS.
Myleran Description
Myleran (busulfan) is a bifunctional alkylating agent. Busulfan is known chemically as 1,4-butanediol dimethanesulfonate and has the following structural formula:
CH3SO2O(CH2)4OSO2CH3
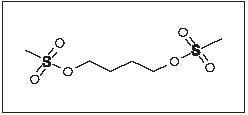
Busulfan is not a structural analog of the nitrogen mustards. Myleran is available in tablet form for oral administration. Each film-coated tablet contains 2 mg busulfan and the inactive ingredients hypromellose, lactose (anhydrous), magnesium stearate, pregelatinized starch, triacetin, and titanium dioxide.
The activity of busulfan in chronic myelogenous leukemia was first reported by D.A.G. Galton in 1953.
Myleran - Clinical Pharmacology
Busulfan is a small, highly lipophilic molecule that easily crosses the blood brain barrier. Following absorption, 32% and 47% of busulfan are bound to plasma...



