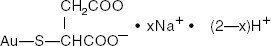Myochrysine
Generic name:gold sodium thiomalate
Dosage form: injection
Drug class:Antirheumatics
Medically reviewed by Drugs.com. Last updated on Mar 22, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Rx only
Physicians planning to use Gold Sodium Thiomalate should thoroughly familiarize themselves with its toxicity and its benefits. The possibility of toxic reactions should always be explained to the patient before starting therapy. Patients should be warned to report promptly any symptoms suggesting toxicity. Before each injection of Gold Sodium Thiomalate, the physician should review the results of laboratory work, and see the patient to determine the presence or absence of adverse reactions since some of these can be severe or even fatal.
DESCRIPTION
Myochrysine® Gold Sodium Thiomalate is a sterile aqueous solution. It contains 0.5 percent BENZYL alcohol added as a preservative. The pH of the product is 5.8 t o 6.5.
Gold Sodium Thiomalate is a mixture of the mono- and di- sodium salts of gold thiomalic acid. The structural formula is:
mercaptobutanedioic acid, monogold (1 +) sodium salt
The molecular weight for C4H3AuNa2O4S (the disodium salt) is 390.07 and for C4H4AuNaO4S (the mono- sodium salt) is 368.09.
Gold Sodium Thiomalate is supplied as a solution for intramuscular injection containing 50 mg of Gold Sodium Thiomalate per mL.
CLINICAL PHARMACOLOGY
The mode of action of Gold Sodium Thiomalate is unknown. The predominant action appears to be a suppressive effect on the synovitis of active rheumatoid disease.