Myzilra
Generic name:levonorgestrel and ethinyl estradiol
Dosage form: tablets
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on Aug 23, 2021.
On This Page
Myzilra Description
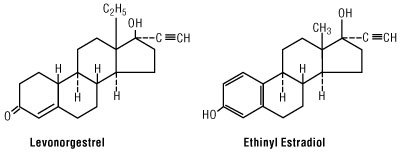
Each Myzilra® cycle of 28 tablets consists of three different drug phases as follows: Phase 1 comprised of 6 beige tablets, each containing 0.05 mg of levonorgestrel (d(-)-13 beta-ethyl-17-alpha-ethinyl-17-beta-hydroxygon-4-en-3-one), a totally synthetic progestogen, and 0.03 mg of ethinyl estradiol (19-nor-17α-pregna-1,3,5(10)-trien-20-yne-3, 17-diol); phase 2 comprised of 5 white tablets, each containing 0.075 mg levonorgestrel and 0.04 mg ethinyl estradiol; and phase 3 comprised of 10 light-yellow tablets, each containing 0.125 mg levonorgestrel and 0.03 mg ethinyl estradiol; then followed by 7 light-green inert tablets. The inactive ingredients present are microcrystalline cellulose, FD&C Blue 2, FD&C Red 40, FD&C Yellow 6, D&C Yellow 10, hypromellose, iron oxide yellow, lactose monohydrate, magnesium stearate, polyethylene glycol, pregelatinized starch, and vitamin E.
Myzilra - Clinical Pharmacology
Combination oral contraceptives primarily act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).
Indications and Usage for Myzilra
Oral contraceptives are indicated for the prevention of pregnancy in women who elect to use Myzilra® Tablets as a method of contraception. Oral contraceptives are highly effective. TABLE I lists the typical accidental pregnancy rates for users of combination oral contraceptives and other methods of contraception. The efficacy of these contraceptive methods, except sterilization and the IUD, depends upon the reliability with which they are used....



