Nasarel
Generic name:flunisolide
Dosage form: nasal spray
Drug class:Nasal steroids
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
On This Page
The Nasarel brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Nasarel Description
Flunisolide, the active component of Nasarel nasal spray, is an anti-inflammatory glucocorticosteroid with the chemical name: 6β-fluoro-11β, 16α, 17, 21 tetrahydroxypregna-1, 4-diene-3, 20-dione cyclic 16, 17-acetal with acetone, hemihydrate. It has the following chemical structure:
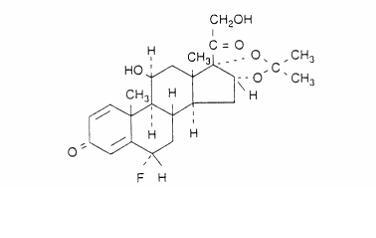
Flunisolide is a white to creamy white crystalline powder with a molecular weight of 443.51 and molecular formula of C24H31FO6. It is soluble in acetone, sparingly soluble in chloroform, slightly soluble in methanol, and practically insoluble in water. It has a melting point of about 245°C.The octanol: water partition coefficient is 2.17 at neutral pH.
Nasarel is a metered dose manual pump spray unit containing a solution of 0.025% w/w flunisolide in an aqueous medium containing benzalkonium chloride, butylated hydroxytoluene, citric acid, edetate disodium, polyethylene glycol 400, polysorbate 20, propylene glycol, sodium citrate dihydrate, sorbitol and purified water. Sodium hydroxide and/or hydrochloric acid may be added to adjust the pH to a target of 5.2. Each 25 mL spray bottle contains 6.25 mg of flunisolide.
After initial priming (5 to 6 sprays), each spray of the pump spray unit delivers a metered spray of 116 mg formulation containing 29 mcg of flunisolide. The size of 99.5% of the droplets produced by the unit is greater than 8 microns. The contents of one nasal spray bottle delivers 200 sprays in addition to the priming sprays.
CLINICAL PHARMACOLOGY:
General Pharmacology
Flunisolide nasal spray has demonstrated potent glucocorticoid and weak mineralocorticoid activity in ...



