NegGram
Generic name:nalidixic acid
Dosage form: tablet
Drug classes:Quinolones, Urinary anti-infectives
Medically reviewed by Drugs.com. Last updated on Mar 22, 2022.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of NegGram (nalidixic acid, USP) and other antibacterial drugs, NegGram should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
On This Page
NegGram Description
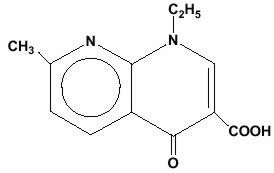
NegGram, brand of nalidixic acid, is a quinolone antibacterial agent for oral administration. Nalidixic acid is 1-ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylic acid. It is a pale yellow, crystalline substance and a very weak organic acid.
Nalidixic acid has the following structural formula:
Inactive Ingredients - Hydrogenated Vegetable Oil, Methylcellulose, Microcrystalline Cellulose, Sodium Lauryl Sulfate, Yellow Ferric Oxide.
NegGram - Clinical Pharmacology
Following oral administration, NegGram is rapidly absorbed from the gastrointestinal tract, partially metabolized in the liver, and rapidly excreted through the kidneys. Unchanged nalidixic acid appears in the urine along with an active metabolite, hydroxynalidixic acid, which has antibacterial activity similar to that of nalidixic acid. Other metabolites include glucuronic acid conjugates of nalidixic acid and hydroxy nalidixic acid, and the dicarboxylic acid derivative. The hydroxy metabolite represents 30 percent of the biologically active drug in the blood and 85 percent in the urine. Peak serum levels of active drug average approximately 20 mcg to 40 mcg per mL (90 percent protein bound), one to two hours after administration of a 1 g dose to a fasting normal individual, with a half-life of about 90 minutes. Peak urine levels of active drug average approximately 150 mcg to 200 mcg per mL, three to four hours after administration, with a half-life of about six hours. Approximately four percent of NegGram is excreted in the feces. Traces of nalidixic acid were found in blood and urine of an infant whose mother had received the drug during the last trimester of pregnancy. (See PRECAUTIONS—Drug Interactions.)



