Nuvakaan II
Generic name:lidocaine and prilocaine
Dosage form: kit
Drug class:Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Oct 1, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
Nuvakaan II Description
Lidocaine 2.5% and Prilocaine 2.5% Cream, USP is an emulsion in which the oil phase is a eutectic mixture of lidocaine and prilocaine cream in a ratio of 1:1 by weight. This eutectic mixture has a melting point below room temperature and therefore both local anesthetics exist as a liquid oil rather than as crystals. It is packaged in 5 gram and 30 gram tubes.
Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), has an octanol: water partition ratio of 43 at pH 7.4, and has the following structure:
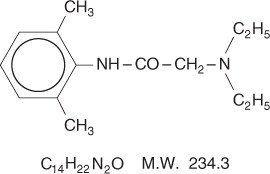
Prilocaine is chemically designated as propanamide, N-(2-methylphenyl)-2-(propylamino), has an octanol: water partition ratio of 25 at pH 7.4, and has the following structure:
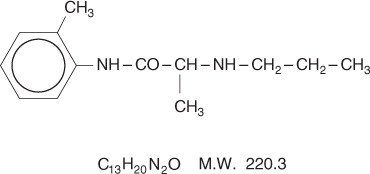
Each gram of lidocaine and prilocaine cream contains lidocaine 25 mg, prilocaine 25 mg, polyoxyethylene fatty acid esters (as emulsifiers), carboxypolymethylene (as a thickening agent), sodium hydroxide to adjust to a pH approximating 9, and purified water to 1 gram. Lidocaine and prilocaine cream contains no preservative, however it passes the USP antimicrobial effectiveness test due to the pH. The specific gravity of lidocaine and prilocaine cream is 1.00.
Nuvakaan II - Clinical Pharmacology
Mechanism of Action: Lidocaine and prilocaine cream applied to intact skin under occlusive dressing, provides dermal analgesia by the release of lidocaine and prilocaine from the cream into...



