Omnitrenidol Injection System
Generic name: lidocaine hydrochloride, triamcinolone acetonide
Dosage form: kit
On This Page
LIDOCAINE HYDROCHLORIDE- lidocaine hydrochloride injection, solution
AQUEOUS SOLUTIONS FOR
INFILTRATION AND NERVE BLOCK
Ampul
Plastic Multiple-dose Fliptop Vial
Glass Teartop Vial
Rx only
Omnitrenidol Injection System Description
Lidocaine Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of lidocaine hydrochloride in water for injection for parenteral administration in various concentrations with characteristics as follows:
| Concentration | 0.5% | 1% | 1.5% | 2% |
| mg/mL lidocaine HCl (anhyd.) | 5 | 10 | 15 | 20 |
| mg/mL sodium chloride | 8 | 7 | 6.5 | 6 |
Multiple-dose vials contain 0.1% of methylparaben added as preservative. May contain sodium hydroxide and/or hydrochloric acid for pH adjustment. The pH is 6.5 (5.0 to 7.0). See HOW SUPPLIED section for various sizes and strengths.
Lidocaine is a local anesthetic of the amide type.
Lidocaine Hydrochloride, USP is chemically designated 2-(diethylamino)-N-(2,6-dimethylphenyl)-acetamide monohydrochloride monohydrate, a white powder freely soluble in water. The molecular weight is 288.82. It has the following structural formula:
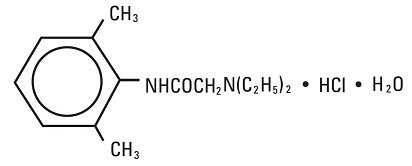
The semi-rigid vial used for the plastic vials is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The container requires no vapor barrier to maintain the proper drug concentration.



