Antitussive Hydrocodone and Homatropine
Generic name: hydrocodone bitartrate and homatropine methylbromide
Dosage form: tablet
Drug class:Upper respiratory combinations
Medically reviewed by Drugs.com. Last updated on Mar 22, 2022.
Rx Only
On This Page
Antitussive Hydrocodone and Homatropine Description
Hydrocodone Bitartrate and Homatropine Methylbromide Tablet, USP contains hydrocodone
(dihydrocodeinone) bitartrate, a semi synthetic centrally-acting opioid antitussive. Homatropine methylbromide is included in a subtherapeutic amount to discourage deliberate overdosage.
Each Hydrocodone Bitartrate and Homatropine Methylbromide Tablet contains:
Hydrocodone Bitartrate, USP 5 mg
Homatropine Methylbromide, USP 1.5 mg
Hydrocodone Bitartrate and Homatropine Methylbromide Tablets also contain: anhydrous lactose, dicalcium phosphate anhydrous, sodium starch glycolate, colloidal silicon dioxide and magnesium stearate.
The hydrocodone component is 4,5α-epoxy-3-methoxy-17-methylmorphinan-6-one tartrate
(1:1) hydrate (2:5), a fine white crystal or crystalline powder which is derived from the opium alkaloid, thebaine, has a molecular weight of (494.50) and may be represented by the following structural formula:
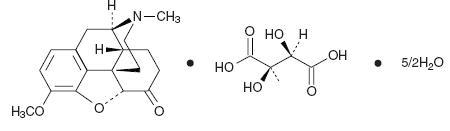
C18H21NO3• C4H6O6• 2½H2O
Hydrocodone Bitartrate
Homatropine methylbromide is 8- Azoniabicyclo[3.2.1]octane, 3-[(hydroxyphenyl-acetyl)oxy]-
8, 8-dimethyl-, bromide, endo-; a white crystal or fine white crystalline powder, with a molecular weight of (370.29).
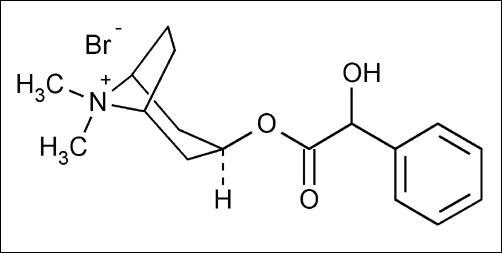
C17H24BrNO3
Homatropine Methylbromide
Antitussive Hydrocodone and Homatropine - Clinical Pharmacology
Hydrocodone is a semisynthetic opioid antitussive and analgesic with multiple actions qualitatively similar to those ...



