Ovcon 35
Generic name:norethindrone and ethinyl estradiol
Dosage form: tablets
Drug classes:Contraceptives, Sex hormone combinations
Medically reviewed by Drugs.com. Last updated on Mar 22, 2022.
On This Page
28-Day Regimen
Rx only
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
The Ovcon 35 brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
DESCRIPTION:
OVCON® 35 28-Day (norethindrone and ethinyl estradiol tablets, USP) provide a continuous regimen for oral contraception derived from 21 light peach tablets composed of norethindrone and ethinyl estradiol to be followed by 7 white tablets of inert ingredients. The structural formulas are:
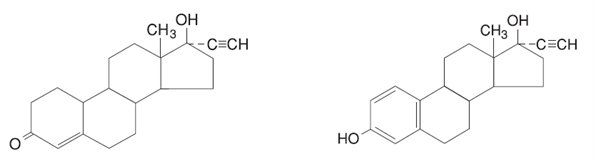
Norethindrone Ethinyl Estradiol
C20H26O2 Molecular Weight: 298.42 C20H24O2 Molecular Weight: 296.40
The light peach active tablets each contain 0.4 mg norethindrone and 0.035 mg ethinyl estradiol, and contain the following inactive ingredients: anhydrous lactose, dibasic calcium phosphate, FD&C yellow no. 6 aluminum lake, lactose monohydrate, magnesium stearate, povidone and sodium starch glycolate. The white tablets in the 28 Day regimen contain only inert ingredients as follows: lactose monohydrate, magnesium stearate, and pregelatinized starch.
CLINICAL PHARMACOLOGY:
Combination oral contraceptives act by suppression of gonadotropins. Although the...



