Oxilan
Generic name: iOxilan
Dosage form: injection, solution
Drug class:Non-ionic iodinated contrast media
Medically reviewed by Drugs.com. Last updated on Feb 21, 2022.
On This Page
Oxilan Description
Oxilan® (IOxilan Injection) formulations are stable, aqueous, sterile, and non- pyrogenic solutions for intravascular administration as diagnostic radiopaque media. IOxilan is designated chemically as N-(2,3-dihydroxypropyl)-N’-(2- hydroxyethyl)-5-[N-(2,3-dihydroxypropyl) acetamido]-2,4,6-triiodoisophthal-amide and has the following structural formula:
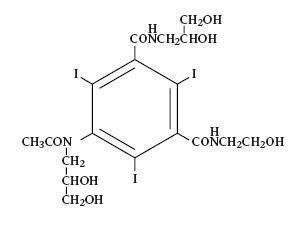
The molecular weight of iOxilan is 791.12 and the organically bound iodine content is 48.1%. IOxilan is nonionic and does not dissociate in solution. Oxilan® (IOxilan Injection) Pharmacy Bulk Package is available in two strengths: Oxilan® (IOxilan Injection) 300 mgI/mL and Oxilan® (IOxilan Injection) 350 mgI/mL.
Each mL of Oxilan® (IOxilan Injection) 300 mgI/mL provides 623 mg iOxilan. Each mL of Oxilan® (IOxilan Injection) 350 mgI/mL provides 727 mg iOxilan. Each mL of Oxilan® (IOxilan Injection) 300 mgI/mL Pharmacy Bulk Package provides 623 mg iOxilan.
Each mL of Oxilan® (IOxilan Injection) 350 mgI/mL Pharmacy Bulk Package provides 727 mg iOxilan.
Each mL of Oxilan® solution contains 0.1 mg edetate calcium disodium (anhydrous basis), 1.0 mg tromethamine, 0.5 mg sodium chloride and a minimum of 0.2 mg (0.01 mEq) sodium. The pH is adjusted to 6.8 (5.5 to 7.5) with hydrochloric acid and sodium hydroxide. The solutions contain no preservative.
Pertinent physicochemical data are below. Oxilan® (IOxilan Injection) is hypertonic compared to plasma (approximately 285 mOsm/kg water).
| Concentration (mgI/mL) |
MEDICAL DEPARTMENTS
Cardiology
Pediatrics
Diabetes Care
Pre-natal Care
Ultrasound Echocardiogram
| |



