Petrem
Generic name: sevoflurane inhalation anesthetic
Dosage form: FOR ANIMAL USE ONLY
Petrem Description
Inhalation Anesthetic For Use in Dogs
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
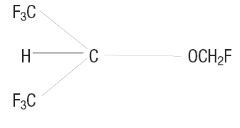
Petrem® (sevoflurane, USP), a volatile liquid, is a halogenated general inhalation anesthetic drug. Its chemical name is fluoromethyl 2,2,2-trifluoro-1-(trifluoromethyl) ethyl ether, and its structural formula is:
Sevoflurane Physical Constants are:
Molecular weight 200.05
Boiling point at 760 mm Hg 58.6°C
Specific gravity at 20°C 1.520-1.525 g/mL
Vapor pressure in mm Hg at 20°C 157
at 25°C 197
at 36°C 317
Distribution Partition Coefficients at 37°C:
Blood/Gas 0.63-0.69
Water/Gas 0.36
Olive Oil/Gas 47-54
Brain/Gas 1.15
Mean Component/Gas Partition Coefficients at 25°C for Polymers Used Commonly in Medical Applications:
Conductive rubber 14.0
Butyl rubber 7.7
Polyvinyl chloride 17.4
Polyethylene 1.3
Sevoflurane is nonflammable and nonexplosive as defined by the requirements of International Electrotechnical Commission 601-2-13.
Sevoflurane is a clear, colorless, stable liquid containing no additives or chemical stabilizers. Sevoflurane is nonpungent. It is miscible with ethanol, ether, chloroform and petroleum benzene, and it is slightly soluble in water. Sevoflurane is stable when stored under normal room lighting condition according to instructions.
INDICATIONS
Petrem is indicated for induction and maintenance of general anesthesia in dogs.
Petrem Dosage and Administration
Inspired Concentration: The delivered concentration of Petrem should be known. Since the depth of anesthesia may be altered easily and rapidly, only vaporizers producing predictable percentage concentrations of sevoflurane should be used. Sevoflurane should be vaporized using a precision vaporizer specifically calibrated for sevoflurane. Sevoflurane contains no stabilizer. Nothing in the drug product alters calibration or operation of these vaporizers. The administration of general anesthesia must be individualized based on the patient's response. WHEN USING SEVOFLURANE PATIENTS SHOULD BE CONTINUOSLY MONITORED AND FACILITIES FOR MAINTENANCE OF PATENT AIRWAY, ARTIFICIAL VENTILATION, AND OXYGEN SUPPLEMENTATION MUST BE IMMEDIATELY AVAILABLE.
Replacement of Desiccated CO2 Absorbents: When a clinician suspects that the CO2



