Podofilox
Dosage form: topical solution
Drug class:Topical keratolytics
Medically reviewed by Drugs.com. Last updated on Mar 22, 2022.
On This Page
Rx only
Podofilox Description
Podofilox Topical Solution 0.5% is an antimitotic drug which can be chemically synthesized or purified from the plant families Coniferae and Berberidaceae (e.g. species of Juniperus and Podophyllum). Podofilox Topical Solution 0.5% is formulated for topical administration. Each milliliter of solution contains 5 mg of Podofilox, in a vehicle containing lactic acid and sodium lactate in alcohol USP 95% (v/v).
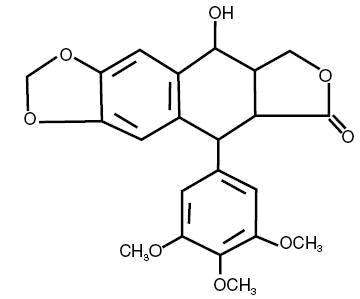
Podofilox has a molecular weight of 414.4 daltons, and is soluble in alcohol and sparingly soluble in water. Its chemical name is 5,8,8a,9-Tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)furo[3',4':6,7] naphtho[2,3,d]-1, 3-dioxol-6(5aH)-one. Podofilox has the following structural formula:
Podofilox - Clinical Pharmacology
Mechanism of Action
Treatment of genital warts with Podofilox results in necrosis of visible wart tissue. The exact mechanism of action is unknown.
Pharmacokinetics
In systemic absorption studies in 52 patients, topical application of 0.05 mL of 0.5% Podofilox solution to external genitalia did not result in detectable serum levels. Applications of 0.1 to 1.5 mL resulted in peak serum levels of 1 to 17 ng/mL one to two hours after application. The elimination half-life ranged from 1.0 to 4.5 hours. The drug was not found to accumulate after multiple treatments.
Clinical Studies
In clinical studies with Podofilox solution, the test product and its vehicle were applied in a double-blind fashion to comparable patient groups. Patients were treated for two to four weeks, and reevaluated at a two-week follow-up examination. Although the number of patients and warts evaluated at eac...