Polmon
Generic name:dexchlorpheniramine maleate
Dosage form: oral solution
Drug class:Antihistamines
Medically reviewed by Drugs.com. Last updated on Sep 1, 2021.
On This Page
Polmon Description
Each 5 mL (teaspoonful) contains:
Dexchlorpheniramine Maleate, USP ..... 2 mg
Dexchlorpheniramine Maleate, USP, an antihistamine agent, is a white, odorless crystalline powder that is freely soluble in water. The molecular formula is C 16H 19ClN 2 • C 4H 4O 4, designated chemically as (+)-2-[p-Chloro-α-[2-(dimethylamino)ethyl]benzyl]pyridine maleate (1:1).
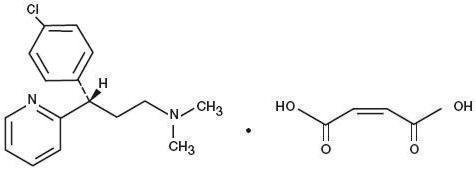
M.W. = 390.86
INACTIVE INGREDIENTS
Citric acid, cherry flavoring, FD&C Red No. 40, glycerin, menthol, methylparaben, propylene glycol, propylparaben, purified water, sodium citrate dihydrate, and sugar.
Polmon - Clinical Pharmacology
Dexchlorpheniramine maleate is an antihistamine with anticholinergic (drying) and sedative side effects. Antihistamines appear to compete with histamine for cell receptor sites on effector cells.
Indications and Usage for Polmon
Perennial and seasonal allergic rhinitis
Vasomotor rhinitis
Allergic conjunctivitis due to inhalant allergens and foods
Mild, uncomplicated allergic skin manifestations of urticaria and angioedema
Amelioration of allergic reactions to blood or plasma
Dermographism
As therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled.
Contraindications
Use in Newborn or Premature Infants
This drug should not be used in newborn or premature infants.
Use in Nursing Mothers
Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.
Use in Lower Respiratory Disease
Antihistamines should NOT be used to treat lower respiratory tract symptoms including asthma.
Antihistamines are al



