Procanbid
Generic name:procainamide hydrochloride
Dosage form: Extended Release Tablets
Drug class:Group I antiarrhythmics
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
On This Page
*Procanbid® is not USP for dissolution.
Positive ANA Titer: The prolonged administration of procainamide often leads to the development of a positive antinuclear antibody (ANA) test, with or without symptoms of a lupus erythematosus-like syndrome. If a positive ANA titer develops, the benefits versus risks of continued procainamide therapy should be assessed.
The Procanbid brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
DESCRIPTION
Procanbid® (Procainamide Hydrochloride Extended-Release Tablets), a Group 1A cardiac antiarrhythmic drug, is p-amino-N-[2-(diethylamino) ethyl] benzamide monohydrochloride, molecular weight 271.79. Its structural formula is:
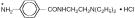
(*Site of acetylation to N-acetylprocainamide)
Procainamide hydrochloride differs from procaine which is the p-aminobenzoyl ester of 2-(diethylamino)-ethanol. Procainamide as the free base has a pKa of 9.24; the monohydrochloride is very soluble in water.
Procanbid® (Procainamide Hydrochloride Extended-Release Tablets) contains 500 mg or 1000 mg of procainamide hydrochloride for oral administration. The release of procainamide hydrochloride is controlled by 2 mechanisms using patented technology. The core of the tablet consists of a wax matrix which is then coated with a polymeric, control-release layer. Both strengths of Procanbid® contain this Polymatrix™ core. Both strengths of Procanbid® contain black iron oxide; candelilla wax, FCC; carnauba wax, NF; colloidal silicon dioxide, NF; hydroxypropyl cellulose, NF; hydroxypropylmethyl cellulose; magnesium ste...



