Pronestyl-SR
Generic name:procainamide hydrochloride
Dosage form: Extended-Release Tablets
Drug class:Group I antiarrhythmics
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
On This Page
The prolonged administration of procainamide often leads to the development of a positive anti-nuclear antibody (ANA) test, with or without symptoms of a lupus erythematosus-like syndrome. If a positive ANA titer develops, the benefits versus risk of continued procainamide therapy should be assessed.
The Pronestyl-SR brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Pronestyl-SR Description
PRONESTYL (procainamide hydrochloride), a Group 1A cardiac antiarrhythmic drug, is p-amino-N-[2-(diethylamino)ethyl]-benzamide monohydrochloride, molecular weight 271.79; its graphic formula is:
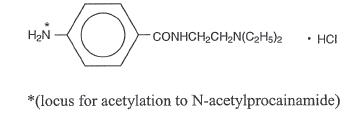
It differs from procaine which is the p-aminobenzoyl ester of 2-(diethylamino)-ethanol. Procainamide as the free base has a pKa of 9.23; the monohydrochloride is very soluble in water.
Pronestyl-SR Tablets (Procainamide Hydrochloride Extended-Release Tablets) for oral administration contain 500 mg procainamide hydrochloride. Inactive ingredients: microcrystalline cellulose, colorants (D&C Yellow No. 10; FD&C Blue No. 2), silicon dioxide, stearic acid, and other ingredients.
Pronestyl-SR - Clinical Pharmacology
Procainamide (PA) increases the effective refractory period of the atria, and to a lesser extent the bundle of His-Purkinje system and ventricles of the heart. It reduces impulse conduction velocity in the atria, His-Purkinje fibers, and ventricular muscle, but has variable effects on the atrioventricular (A-V) node, a direct slowing action and a weaker vagolytic effect which may speed A-V conduction slightly. Myocardial excitability is reduced in the atria, Purkinje fibers, papillary muscles, and ven...



