Prostin VR Pediatric
Generic name:alprostadil
Dosage form: injection, solution
Drug classes:Impotence agents, Vasodilators
Medically reviewed by Drugs.com. Last updated on Apr 21, 2022.
On This Page
500 micrograms per mL
Apnea is experienced by about 10 to 12% of neonates with congenital heart defects treated with Prostin VR Pediatric Sterile Solution. Apnea is most often seen in neonates weighing less than 2 kg at birth and usually appears during the first hour of drug infusion. Therefore, respiratory status should be monitored throughout treatment, and Prostin VR Pediatric should be used where ventilatory assistance is immediately available.
Prostin VR Pediatric Description
Prostin VR Pediatric Sterile Solution for intravascular infusion contains 500 micrograms alprostadil, more commonly known as prostaglandin E1, in 1.0 mL dehydrated alcohol.
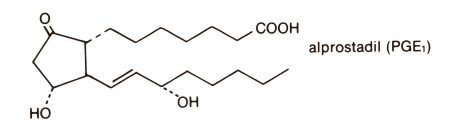
The chemical name for alprostadil is (11α,13E,15S)-11,15 dihydroxy-9-oxo-prost-13-en-1-oic acid, and the molecular weight is 354.49.
Alprostadil is a white to off-white crystalline powder with a melting point between 110° and 116°C. Its solubility at 35°C is 8000 micrograms per 100 mL double distilled water.
Structural Formula
Prostin VR Pediatric - Clinical Pharmacology
Alprostadil (prostaglandin E1) is one of a family of naturally occurring acidic lipids with various pharmacologic effects. Vasodilation, inhibition of platelet aggregation, and stimulation of intestinal and uterine smooth muscle are among the most notable of these effects. Intravenous doses of 1 to 10 micrograms of alprostadil per kilogram of body weight lower the blood pressure in mammals by decreasing peripheral resistance. Reflex increases in cardiac output and rate accompany the reduction in blood pressure.
Smooth muscle of the ductus arteriosus is especially sensitive to alprostadil, and strips of lamb ductus markedly relax in the presence of the drug. In addition, administration of alprostadil reopened the c...



