Quinja Gel
Generic name: iodoquinol and aloe polysaccharide
Dosage form: gel
Drug class:Topical anti-infectives
Medically reviewed by Drugs.com. Last updated on Nov 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Prescribing Information
QUINJA™ gel
(1.25% iodoquinol and 1% aloe polysaccharides)
Quinja Gel Description
Each gram of QUINJA contains 1.25% (12.5 mg) Iodoquinol and 1% (10 mg) Aloe Polysaccharides. Other ingredients: Purified Water, Carbomer 980, Magnesium Aluminum Silicate, PEG-20 Methyl Glucose Ether, Aminomethyl Propanol 95, Biopeptide, Propylene Glycol, Glycerine, SDA Alcohol 40 B, Benzyl Alcohol, Trolamine, FD&C Blue #1 and D&C Yellow #10.
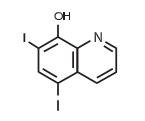
Iodoquinol
Iodoquinol is an antifungal and antibacterial agent. Chemically, Iodoquinol is [5,7-diiodo-8-quinolinol] with the molecular formula (C9H5I2NO) and is represented by the following structural formula:
Aloe Polysaccharide
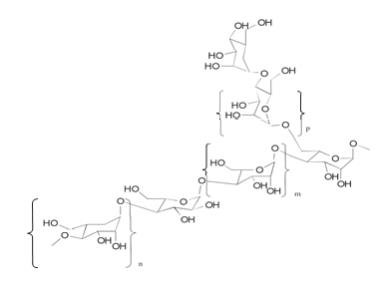
The Aloe Polysaccharide in QUINJA is a patented mixture of acetylated mannan aloe polysaccharide mixture with average molecular weights of 80 and 1300 kDa (CAS 89191- 46-8). Each purified acetylated mannan polysaccharide of specific molecular weight range and average is composed of the same repeating subunits shown below (where m is mannose, n is galactose and p is glucose monomers):
Indications and Usage for Quinja Gel
Based on a review of a related drug by the National Research Council and subsequent FDA classification for that drug, the indications are as follows: “Possibly” Effective: Contact or atopic dermatitis; impetiginized eczema; nummular eczema; endogenous chronic infectious dermatitis; stasis dermatitis; pyoderma; nuchal eczema and chronic eczematoid otitis externa; acne urticata; localized or disseminated neurodermatitis; lichen simplex chronicus; anogenital pruritus (vulvae, scroti, ani); folliculitis; bacterial dermatoses; mycotic dermatoses such as tinea (capitis, cruris, corporis, pedis); moniliasis; intertrigo. Final classification of the less-than- effective indications requires further investi...



