Generic name: ranitidine hydrochloride
Dosage form: injection, solution
Drug class:H2 antagonists
Medically reviewed by Drugs.com. Last updated on Nov 1, 2021.
On This Page
Ranitidine Injection Description
The active ingredient in Ranitidine Injection is ranitidine hydrochloride (HCl), a histamine H2-receptor antagonist. Chemically it is N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N′-methyl-2-nitro-1,1-ethenediamine, hydrochloride. It has the following structure:
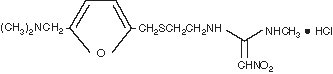
The empirical formula is C13H22N4O3S●HCl, representing a molecular weight of 350.87.
Ranitidine hydrochloride USP is a white to pale yellow, crystalline powder that is very soluble in water.
Ranitidine Injection USP is a clear, colorless to yellow, nonpyrogenic liquid. The yellow color of the liquid tends to intensify without adversely affecting potency. The pH of the injection solution is 6.7 to 7.3.
Each 1 mL of aqueous solution contains ranitidine 25 mg (as the hydrochloride); phenol 5 mg as preservative; and 0.96 mg of monobasic potassium phosphate and 2.4 mg of dibasic sodium phosphate as buffers.
A pharmacy bulk package is a container of a sterile preparation for parenteral use that c...



