Rescon JR
Generic name:phenylephrine hydrochloride and dexchlorpheniramine maleate
Dosage form: tablet, multilayer, extended release
Drug class:Upper respiratory combinations
Medically reviewed by Drugs.com. Last updated on Dec 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
The Rescon-Jr brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Rescon JR Description
Nasal Decongestant / Antihistamine, sustained-release tablets for oral administration.
Each RESCON-Jr.® tablet contains:
Phenylephrine Hydrochloride 20 mg
Dexchlorpheniramine Maleate 3 mg
Phenylephrine HCI is a sympathomimetic amine with the chemical structure: Benzenemethanol, 3- hydroxy-(- [(methylamino)methyl]- hydrochloride.
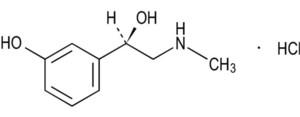
C9H13NO2 • HCI M.W. 203.67
Dexchlorpheniramine Maleate is an antihistamine with the chemical structure: (+)-2-[p-Chloro-(- [2-(dimethylamino) ethyl]benzyl] pyridine maleate (1:1)
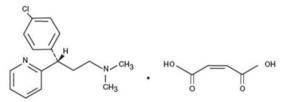
C16H19CIN2 • C4H4O4 M.W. 390.86
INACTIVE INGREDIENTS
calcium phosphate dibasic, D&C Yellow #10, magnesium stearate (veg.), methylcellulose, povidone, and silicified microcrystalline cellulose.



