Dosage form: tablet, film coated
Drug class:H2 antagonists
Medically reviewed by Drugs.com. Last updated on Dec 22, 2021.
On This Page
Ranitidine Tablets Description
The active ingredient in Ranitidine Tablets USP, 150 mg and Ranitidine Tablets USP, 300 mg is ranitidine hydrochloride (HCl) USP, a histamine H2-receptor antagonist. Chemically it is N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N′-methyl-2-nitro-1,1-ethenediamine, HCl. It has the following structue:
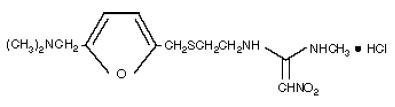
The empirical formula is C13H22N4O3S•HCl, representing a molecular weight of 350.87.
Ranitidine HCl is a white to pale yellow, granular substance that is soluble in water. It has a slightly bitter taste and sulfur like odor.
Each Ranitidine Tablet USP, 150 mg for oral administration contains 168 mg of ranitidine HCl equivalent to 150 mg of ranitidine. Each tablet also contains the inactive ingredients Colloidal Silicon Dioxide (Aerosil 200 NF), Croscarmellose Sodium NF (Ac-Di-Sol), FD&C Yellow #6/ Sunset Yellow FCF Aluminium Lake, glyceryl monostearate, Iron oxide yellow, Magnesium stearate NF (Ligamed MF-2-V), Microcrystalline Cellulose NF (Avicel PH-112), Polyvinyl alcohol,Purified Water USP, Sodium Lauryl Sulfate, Talc and Titanium Dioxide.
Each Ranitidine Tablet USP, 300 mg for oral administration contains 336 mg of ranitidine HCl equivalent to 300 mg of ranitidine. Each tablet also contains the inactive ingredie..



