Rea Lo 39
Generic name:urea
Dosage form: cream
Drug class:Topical emollients
Medically reviewed by Drugs.com. Last updated on Jul 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Rx Only
DESCRIPTION
Rea Lo 39™ (Urea 39%) Cream is a keratolytic emollient, which is a gentle, yet effective, tissue softener for skin. Each gram contains 390 mg Urea as the active ingredient and the following inactive ingredients: purified water, emulsifying wax, glycerin, isopropyl myristate, sorbitol, neopentyl glycol dicaprylate/dicaprate, tridecyl stearate, tridecyl trimellitate and dimethyl isosorbide.
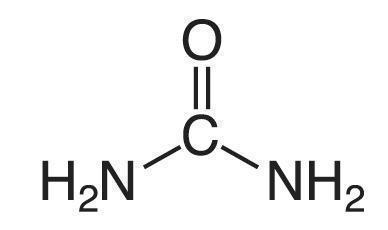
Urea is a diamide of carbonic acid with the following structure:
Rea Lo 39 - Clinical Pharmacology
Urea cream gently dissolves the intracellular matrix which results in loosening of the horny layer of the skin and shedding of scaly skin at regular intervals, thereby softening hyperkeratotic areas of the skin.
PHARMACOKINETICS
The exact mechanism of action of topically applied urea is not known.
INDICATION AND USAGE
Rea Lo 39™ (Urea 39%) Cream is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, xerosis, ichthyosis, skin cracks and fissures, dermatitis, eczema, psoriasis, keratoses and calluses.
Contraindications
Known hypersensitivity to any of the listed ingredients.
Warnings
For external use only. Avoid contact with eyes, lips or mucous membranes.
Precautions
Urea cream should be used as directed by a physician and should not be used to treat conditions other than those for which it was prescribed. If redness or irritation occurs, discontinue use.



