Saluron
Generic name: hydroflumethiazide
Dosage form: Tablets
Drug class:Thiazide diuretics
Medically reviewed by Drugs.com. Last updated on Jun 21, 2021.
On This Page
Description
Saluron® (hydroflumethiazide) is a potent oral diuretic-antihypertensive agent of low toxicity. Each tablet contains 50 mg of hydroflumethiazide.
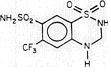
Saluron® is 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 3,4-dihydro-6-(trifluoromethyl)-, 1,1-dioxide. Hydroflumethiazide is very slightly soluble in water, soluble in methanol and freely soluble in acetone. Inactive ingredients: microcrystalline cellulose, lactose, magnesium stearate, colloidal silicon dioxide, and sodium starch glycolate. It has the following structural formula:
Clinical Pharmacology
Hydroflumethiazide is incompletely but fairly rapidly absorbed from the gastrointestinal tract. It appears to have a biphasic biological half-life with an estimated alpha-phase of about 2 hours and an estimated beta-phase of about 17 hours; it has a metabolite with a longer half-life, which is extensively bound to the red blood cells. Hydroflumethiazide is excreted in the urine; its metabolite has also been detected in the urine.
The mechanism of action results in an interference with the renal tubular mechanism of electrolyte reabsorption. At maximal therapeutic dosage, all thiazides are approximately equal in their diuretic potency. The mechanism whereby thiazides function in the control of hypertension is unknown.
Indications and Usage
Saluron® is indicated as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis and corticosteroid and estrogen therapy.
Saluron® has also been found useful in edema due to various forms of renal dysfunction, such as nephrotic syndrome, acute glomerulonephritis, and chronic renal failure.
Saluron® is indicated in the management of hypertension, either as the sole therapeutic agent or to enhance the effectiveness ...



