Sansert
Generic name: methysergide maleate
Dosage form: Tablets, USP
Drug class:Antimigraine agents
Medically reviewed by Drugs.com. Last updated on Jan 24, 2022.
T2000-69
89011201
Information for the physician
Sansert®
(methysergide maleate) tablets, USP
Rx only
Prescribing Information
On This Page
Retroperitoneal Fibrosis, Pleuropulmonary Fibrosis and Fibrotic Thickening of Cardiac Valves May Occur in Patients Receiving Long-term Methysergide Maleate Therapy. Therefore, This Preparation Must Be Reserved for Prophylaxis in Patients Whose Vascular Headaches Are Frequent and/or Severe and Uncontrollable and Who Are Under Close Medical Supervision.
(See also WARNINGS section)
Sansert Description
Sansert® (methysergide maleate) is a partially synthetic compound structurally related to lysergic acid butanolamide, well-known as methylergonovine in obstetrical practice as an oxytocic agent.
Chemically, methysergide maleate is designated as ergoline-8-carboxamide, 9,10-didehydro-N-[1-(hydroxymethyl)propyl]-1,6-dimethyl-, (8ß)-, (Z)-2-butenedioate (1:1) (salt).
Its structural formula is:
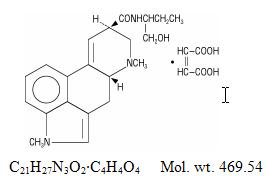
Methylation in the number 1 position of the ring structure enormously enhances the antagonism to serotonin which is present to a much lesser degree in the partially methylated compound (methylergonovine maleate) as well as profoundly altering other pharmacologic properties.
Active Ingredient: methysergide maleate, USP.
Inactive Ingredients: acacia, carnauba wax, colloidal silicon dioxide, FD&C Blue #1, FD&C Yellow #5, gelatin, lactose, malic acid, povidone, sodium benzoate, starch (corn), stearic acid, sucrose, synthetic black iron oxide, talc, and titanium dioxide.
ACTIONS
Sansert® (methysergide maleate) has been shown, in vitro and



