Sinografin
Generic name:diatrizoate meglumine and iodipamide meglumine
Dosage form: injection, solution
Drug class:Ionic iodinated contrast media
Medically reviewed by Drugs.com. Last updated on Nov 22, 2021.
On This Page
Sinografin Description
Sinografin (Diatrizoate Meglumine and lodipamide Meglumine Injection) is a sterile, nonpyrogenic, essentially colorless to pale yellow, aqueous radiopaque contrast medium for intrauterine instillation. Each mL provides 527 mg diatrizoate meglumine and 268 mg iodipamide meglumine with 3.2 mg sodium citrate as a buffer, and 0.4 mg edetate disodium; pH has been adjusted to 7.0 to 7.8 with meglumine and diatrizoic acid. Each mL contains approximately 0.91 mg (0.04 mEq) sodium and 380 mg organically bound iodine. At the time of manufacture, the air in the container is replaced with nitrogen.
Diatrizoate meglumine is designated chemically as 1-deoxy-1-( methylamino)-D-glucitoI 3,5-diacetamido-2,4,6- triiodobenzoate (salt); iodipamide meglumine is 1-deoxy-1- (methylamino)-D-glucitoI 3,3´-(adipoyldiimino)bis[2,4,6- triiodobenzoate] (2:1) (salt). Structural formulas:
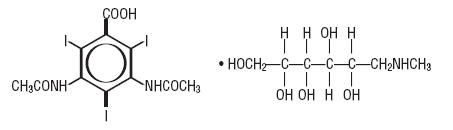
diatrizoate meglumine C11H9I3N2O4• C7H17NO5 MW 809.13
Organically Bound Iodine: 47.1% CAS-131-49-7
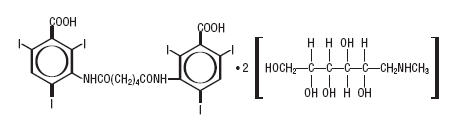
iodipamide meglumine C20H14I6N2O6• 2C7H17NO5 MW 1530.20
Organically Bound Iodine: 49.8% CAS-3521-84-4
Sinografin - Clinical Pharmacology
The most important characteristic of contrast media is the iodine content. The relatively high atomic weight of iodine contributes sufficient radiodensity for radiographic contrast of the uterus and uterine tubes with surrounding tissues.



