Sodium Sulfacetamide Medicated Pads
Dosage form: topical swab
Drug class:Topical antibiotics
Medically reviewed by Drugs.com. Last updated on Jul 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Topical Antibacterial Therapy
FOR DERMATOLOGICAL USE ONLY. NOT FOR OPHTHALMIC USE.
On This Page
DESCRIPTION: 10% Sodium Sulfacetamide Medicated Pads contain 10% sodium sulfacetamide. Inactive ingredients: purified water, sodium thiosulfate, trisodium EDTA, and urea.
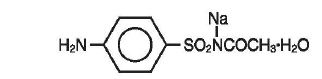
Sodium sulfacetamide is a sulfonamide with antibacterial activity. Chemically, sodium sulfacetamide is C8H9N2Na03S•H20, with a molecular weight of 254.24. Chemically it is Acetamide, N- [(4 aminophenyl) sulfonyl]-, monosodium salt and monohydrate, with the following structural formula:
CLINICAL PHARMACOLOGY: Sodium sulfacetamide exerts a bacteriostatic effect against sulfonamide-sensitive gram-positive and gram-negative microorganisms including Propionibacterium acnes commonly isolated from secondary cutaneous pyogenic infections.
The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory, which is based on the fact that sulfonamides act as competitive antagonists to para-aminobenzoic acid (PABA), an essential component for bacterial growth. While absorption through intact skin in humans has not been determined, sodium sulfacetamide is readily absorbed from the gastrointestinal tract when taken orally and excreted in the urine largely unchanged. The biological half-life has variously been reported as 7 to 12.8 hours.
INDICATIONS AND USAGE: 10% Sodium Sulfacetamide Medicated Pads are indicated for the treatment of bacterial infections of the skin due to organisms susceptible to sulfonamides including Propionibacterium acnes. They are also intended for topical control of seborrheic dermatitis of the face..



