Sulfacetamide Topical
Generic name: sulfacetamide sodium
Dosage form: topical suspension
Drug class:Topical antibiotics
Medically reviewed by Drugs.com. Last updated on Aug 23, 2021.
On This Page
Rx only
FOR TOPICAL USE ONLY.
DESCRIPTION:
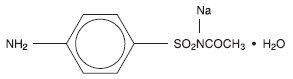
Each mL of Sulfacetamide Sodium Topical Suspension USP, 10% contains 100 mg of sulfacetamide sodium in a vehicle consisting of purified water, propylene glycol, lauramide DEA (and) diethanolamine, polyethylene glycol 400 monolaurate, hydroxyethyl cellulose, sodium chloride, sodium metabisulfite, methylparaben, xanthan gum, EDTA and simethicone. Sulfacetamide sodium is a sulfonamide with antibacterial activity. Chemically sulfacetamide sodium is N'-[(4-aminophenyl) sulfonyl]-acetamide, monosodium salt, monohydrate. The structural formula is:
CLINICAL PHARMACOLOGY:
The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory, based on sulfonamides acting as a competitive inhibitor of para-aminobenzoic acid (PABA) utilization, an essential component for bacterial growth. While absorption through intact skin in humans has not been determined, invitro studies with human cadaver skin indicated a percutaneous absorption of about 4%. Sulfacetamide sodium is readily absorbed from the gastrointestinal tract when taken orally and excreted in the urine largely unchanged. The biological half-life has been reported to be between 7 to 13 hours.
The pharmacokinetics of sulfacetamide and its major metabolite sulfanilamide in Sulfacetamide Sodium Topical Suspension USP, 10% was evaluated in adult subjects (N=14) with acne vulgaris. The subjects applied Sulfacetamide Sodium Topical Suspension USP, 10% to their face, back, chest and shoulders every 12 hours for 28 days. The percentage of the applied dose of Sulfacetamide Sodium Topical Suspension USP, 10% excreted in the urine as sulfacetamide plus sulfanilamide, ranged from 0.08 to 0.33%.
INDICATIONS:
Sulfacetamide Sodium Topical Suspension USP, 10% is indicated in the topical treatment of acne vulgaris.
CONTRAINDICATIONS:
Sulfacetamide Sodium Topical Suspension USP, 10% is contraindicated for use by patients having a known hypersensitivity to sulfonamides or any other component of this preparation (see WARNINGS