Atuss DS Tannate Suspension
Generic name:dextromethorphan hydrobromide, pseudoephedrine hydrochloride and chlorpheniramine maleate
Dosage form: suspension
Drug class:Upper respiratory combinations
Medically reviewed by Drugs.com. Last updated on Jan 24, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
The Atuss DS brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Atuss DS Tannate Suspension Description
Each teaspoonful (5 mL) of Atuss® DS Tannate Suspension contains:
Dextromethorphan Hydrobromide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30 mg
Pseudoephedrine Hydrochloride . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30 mg
Chlorpheniramine Maleate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 mg
Atuss® DS Tannate Suspension is used for oral administration only.
Atuss® DS Tannate Suspension contains the following inactive ingredients: Acesulfame K, Artificial Bubblegum Flavor, Artificial Grape Flavor, Aspartame, Bitter Mask, Citric Acid, FDC Blue #1, FDC Red #40, Glycerin, Hydrochloric Acid, Methylparaben, Magnesium Aluminometasilicate, Purified Water, Sodium Citrate Dihydrate, Sodium Hydroxide, Sucralose, Xanthan Gum. Plus tannic acid yielding a tannate suspension.
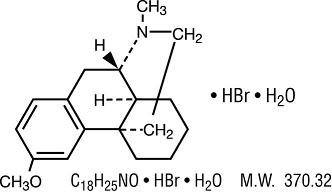
Dextromethorphan Hydrobromide:
3-Methoxy-17-methyl-9α, 13α, 14α-morphinan.

Pseudoephedrine Hydrochloride:
[S(R*, R*)]- α -[1-(meth.



