Sulfatol C Cream
Generic name:sulfacetamide sodium and sulfur
Dosage form: cream
Drug class:Topical antibiotics
Medically reviewed by Drugs.com. Last updated on Apr 21, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Rx Only
The Sulfatol C brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
PRODUCT DESCRIPTION
Sulfatol C™ Cream (sulfacetamide sodium 10% and sulfur 5%) is available by prescription only and contains the following active ingredients: Sulfacetamide Sodium 10% and Sulfur 5%.
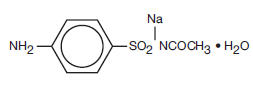
Chemically, sulfacetamide sodium is Acetamide, N-[(4-aminophenyl) sulfonyl], monosodium salt, monohydrate. The structural formula is:
INACTIVE INGREDIENTS: Benzyl Alcohol, Cetyl Alcohol, Cocoglycerides, Dimethicone, Disodium EDTA, Fragrance, Glyceryl and PEG-100 Stearate, Isopropyl Myristate, Light Mineral Oil, Polysorbate 60, Propylene Glycol, Sodium Thiosulfate, Sorbitan Monostearate, Stearyl Alcohol, Urea, Purified Water, Xanthan Gum, Zinc Ricinoleate.
Sulfatol C Cream - Clinical Pharmacology
The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory, which is based on the fact that sulfonamides act as competitive antagonists to para-aminobenzoic acid (PABA), an essential component for bacterial growth. While absorption through intact skin has not been determined, sulfacetamide sodium is readily absorbed from the gastrointestinal tract when taken orally and excreted in the urine, largely unchanged. The biological half-life has variously been reported as 7 to 12.8 hours. The exact mode of action of sulfur in the treatment of acne is unknown, but it has been reported that it inhibits the growth of Propionibacterium acnes and the formation of free fatty acids.
INDICATIONS



