Sumansetron
Generic name: sumatriptan succinate, ondansetron
Dosage form: kit
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
- Description
- Indications and Usage
- Clinical Pharmacology
- Drug Interactions
- Clinical Studies
- Contraindications
- Warnings
- Precautions
- Patient Counseling Information
- Adverse Reactions/Side Effects
- Drug Abuse and Dependence
- Overdosage
- Dosage and Administration
- How Supplied/Storage and Handling
- Warnings and Precautions
- Use In Specific Populations
- Nonclinical Toxicology
Sumansetron Description
Sumatriptan tablets USP contain sumatriptan (as the succinate), a selective 5-hydroxytryptamine 1 receptor subtype agonist. Sumatriptan succinate USP is chemically designated as 3-[2- (dimethylamino) ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and it has the following structure:
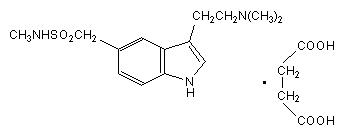
The molecular formula is C 14H 21N 3O 2S•C 4H 6O 4, representing a molecular weight of 413.5. Sumatriptan succinate USP is a white to off-white powder that is readily soluble in water and in saline. Each sumatriptan tablet USP for oral administration contains 35, 70, or 140 mg of sumatriptan succinate USP equivalent to 25, 50, or 100 mg of sumatriptan, respectively. Each tablet also contains the inactive ingredients croscarmellose sodium, lactose anhydrous, lactose monohydrate, magnesium stearate, mannitol, microcrystalline cellulose, talc, titanium dioxide and triacetin.
Sumansetron - Clinical Pharmacology
Mechanism of Action
Sumatriptan is an agonist for a vascular 5-hydroxytryptamine 1 receptor subtype (probably a member of the 5-HT 1D family) having only a weak affinity for 5-HT 1A, 5-HT 5A, and 5-HT



