Sumycin Tablets
Generic name:tetracycline hydrochloride
Dosage form: tablet, film coated
Drug class:Tetracyclines
Medically reviewed by Drugs.com. Last updated on Jul 22, 2021.
On This Page
The Sumycin brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Sumycin Tablets Description
Tetracycline is a yellow, crystalline powder. Tetracycline is soluble in water, slightly soluble in ethanol (96%), practically insoluble in acetone. It dissolves in solutions of alkali hydroxides and carbonates. Solutions in water become turbid on standing, owing to the precipitation of tetracycline. The chemical name for tetracycline hydrochloride is 4-(Dimethylamino)-1,4,4a,5,5a,6,11,12aoctahydro-3,6,10,12,-12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecar-boxamide monohydrochloride. Its structural formula is as follows:
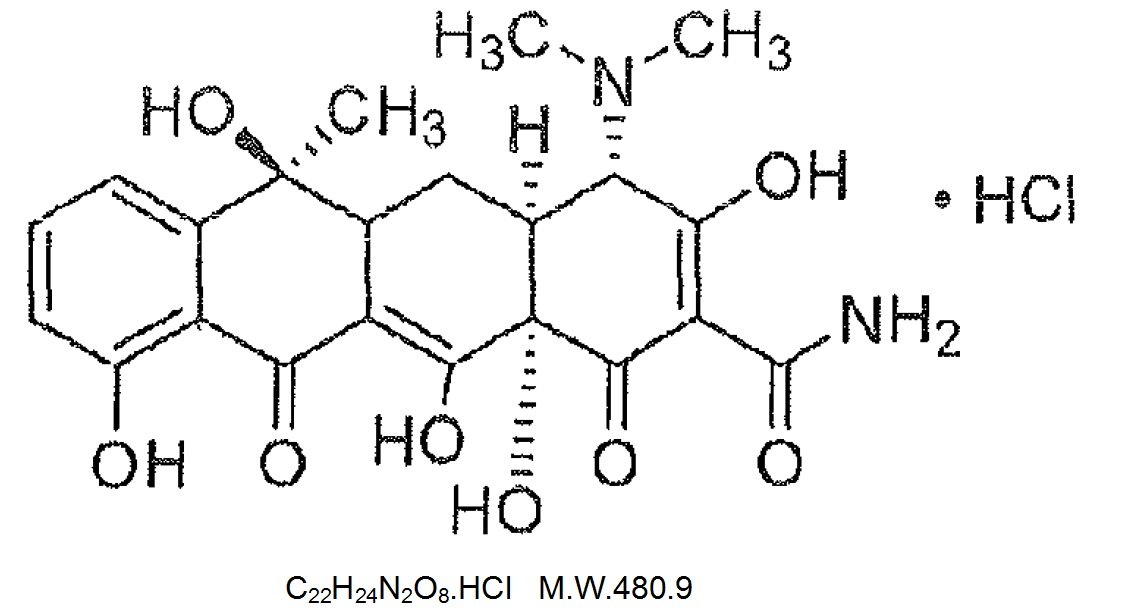
Each tablet, for oral administration, contains 250 mg or 500 mg tetracycline hydrochloride.
Inactive Ingredients: anhydrous lactose, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch and stearic acid. The film coating for the 250 mg and 500 mg are made of D&C RED # 30 / helendon pink aluminium lake, hypromellose and titanium dioxide.
In addition to these, the 250 mg tablet film coating includes triacetin and 500 mg tablet film coating includes polyethylene glycol.
Sumycin Tablets - Clinical Pharmacology
Tetracyclines are readily absorbed and are bound to plasma protein in varying degrees. They are concentrated by the liver in the bile and excreted in the urine and feces at high concentrations in a biologically active form.
Microbiology
Tetracyclines are primarily bacteriostatic and exert their antimicrobial effect by the inhibition of protein synthesis by binding to t...



