SunVanish Cream
Generic name:hydroquinone
Dosage form: cream
Drug class:Topical depigmenting agents
Medically reviewed by Drugs.com. Last updated on Jun 21, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Dosage Apply SunVanish in the morning to the affected area or as directed by your physician.
Warnings Contains sodium Metabisulfite, a sulfite that may cause serious allergic type reactions (eg hives, itching, wheezing, anaphylaxis, severe asthma attack) in certain susceptible persons.
For external use only
Distributed by
Genesis Pharmaceuticals Inc
Parsippany, NJ 07054
Made in USA
18004598663
www.glytone.com
Keep out of reach of children
Contains Each gram of SunVanish contains 40 mg of hydroquinone USP, 75 mg of octinoxate and 50 mg of oxybenzone in a cream base of purified water, proplyene glycol, cetostearyl alcohol, glycerin, isopropyl palmitate, sodium metabisulfite, sodium lauryl sulfate, and sorbic acid
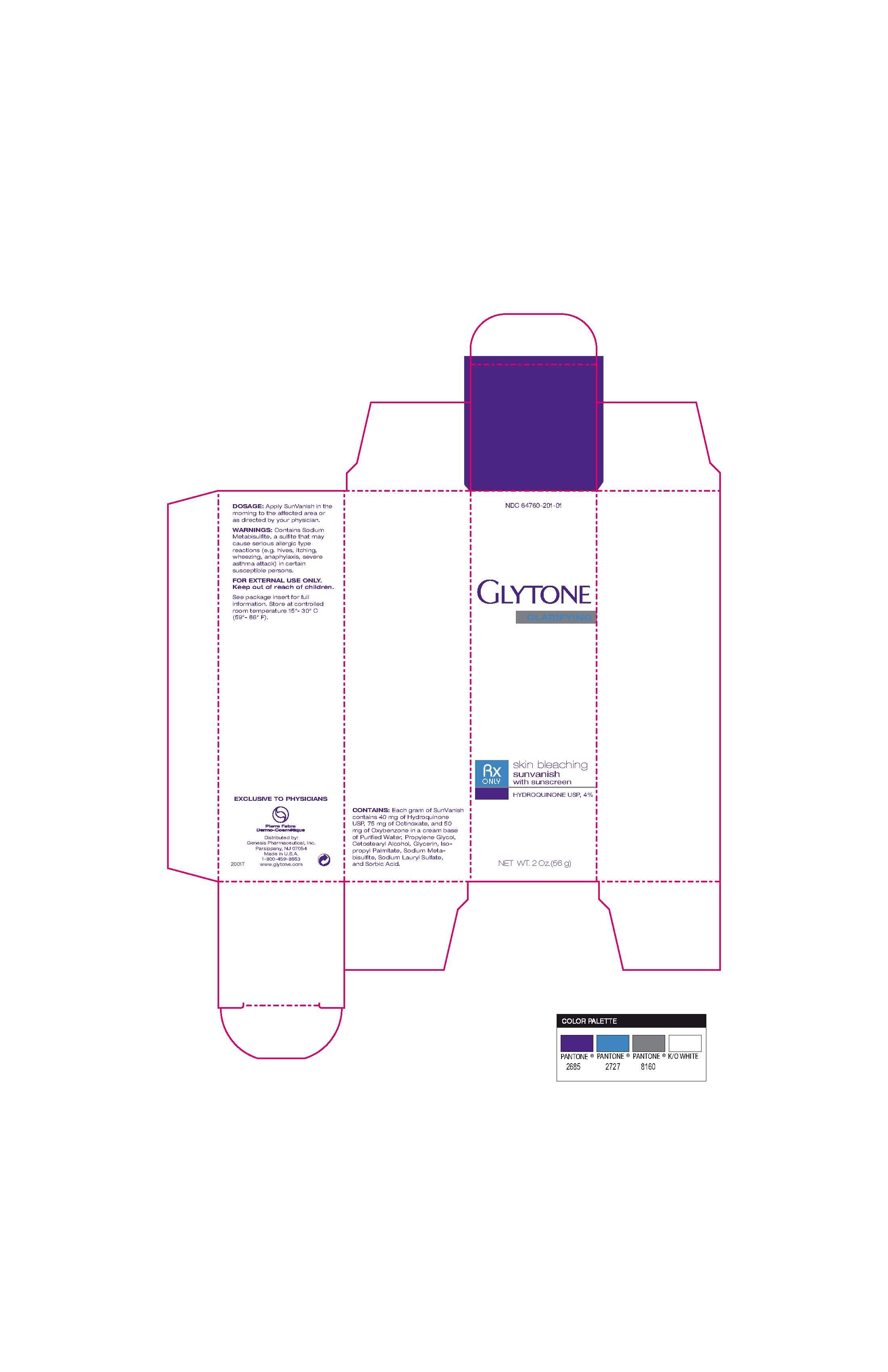
Glytone skin bleaching sunvanish with sunscreen
hydroquinone USP, 4%
RX only
Net wt 2 oz (56 g)
| SUNVANISH hydroquinone cream | ||||||||||||
| ||||||||||||
| ||||||||||||



