Synvexia Patch
Generic name:lidocaine hydrochloride and menthol
Dosage form: patch
Drug class:Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Feb 21, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Warnings
For external use only
Avoid contact with eyes
Do not applly to open wounds or damaged skin
If symptoms persist for more than seven days, discontinue use and consult physician
If swallowed, consult physician
Do not bandage tightly
If pregnant or breast feeding, contact physician prior to use
Do not use in large quantities, particularly over raw surfaces or blistered areas
Keep out of reach of children
Lidocaine HCL 4.00%
Menthol 1.00%
Silicon Dioxide.
clean and dry affected area
remove patch from backing and apply to affected area
use only one patch at a time, and maximum of four patches/day
leave patch on affected area for up to 8 hours
do not use patches for longer than five consecutive days
children under 12 should consult physician prior to use
How Supplied
Use only one patch at a time, and maximum of four patches/day. leave patch on affected area for up to 8 hours
NDC:69336-200-05 5 in 1 BOX
NDC:69336-200-10 10 in 1 BOX
NDC:69336-200-15 15 in 1 BOX
NDC:69336-200-30 30 in 1 BOX
NDC:69336-200-60 60 in 1 BOX
Indications and Usage for Synvexia Patch
Temporary relief of pain associated with minor cuts, scrapes and minor skin irritations
Topical anesthetic
External analgesic
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
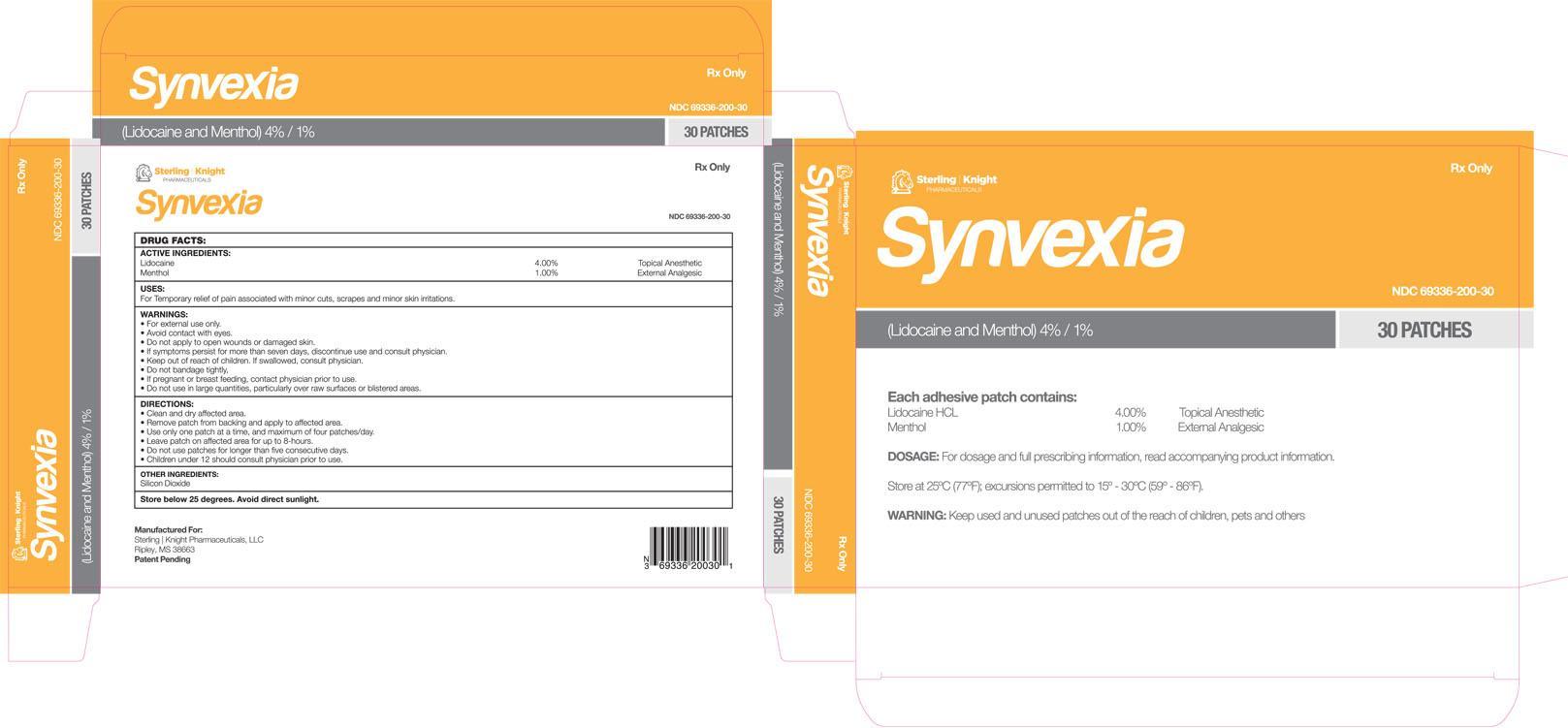
| SYNVEXIA lidocaine hydrochloride and menthol patch | ||||
| ||||



