Teniposide Injection
Dosage form: injection, solution
Drug class:Mitotic inhibitors
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
On This Page
Rx only
WARNING
Teniposide Injection is a cytotoxic drug which should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy and complications is possible only when adequate treatment facilities are readily available.
Severe myelosuppression with resulting infection or bleeding may occur. Hypersensitivity reactions, including anaphylaxis-like symptoms, may occur with initial dosing or at repeated exposure to Teniposide Injection. Epinephrine, with or without corticosteroids and antihistamines, has been employed to alleviate hypersensitivity reaction symptoms.
Teniposide Injection Description
Teniposide Injection is supplied as a sterile nonpyrogenic solution in a nonaqueous medium intended for dilution with a suitable parenteral vehicle prior to intravenous infusion.
Teniposide Injection is available in 50 mg (5 mL) ampules. Each mL contains 10 mg teniposide, 30 mg benzyl alcohol, 60 mg N,N-dimethylacetamide, 500 mg purified polyoxyl 35 castor oil1 and 42.7% (v/v) dehydrated alcohol. The pH of the clear solution is adjusted to approximately 5 with maleic acid.
Teniposide is a semisynthetic derivative of podophyllotoxin. The chemical name for teniposide is 4-demethylepipodophyllotoxin 9-[4,6-O-(R)-2-thenylidene-β-D-glucopyranoside].
Teniposide differs from etoposide, another podophyllotoxin derivative, by the substitution of a thenylidene group on the glucopyranoside ring.
Teniposide has the following structural formula: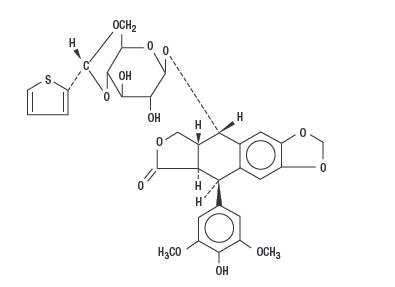
Teniposide is a white to off-white crystalline powder with the empirical formula C32H32O13S and a molecular weight of 656.66. It is a lipophil...



