Terconazole
Dosage form: vaginal suppository
Drug class:Vaginal anti-infectives
Medically reviewed by Drugs.com. Last updated on Oct 1, 2021.
On This Page
Rx Only
Terconazole Description
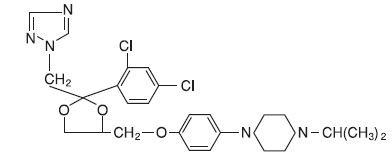
Terconazole Vaginal Suppositories, 80 mg are white to off-white suppositories for intravaginal administration containing 80 mg of the antifungal agent Terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2- (1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4- isopropylpiperazine, in triglycerides derived from coconut and/or palm kernel oil (a base of hydrogenated vegetable oils) and butylated hydroxyanisole.
The structural formula of Terconazole is as follows:
Terconazole, a triazole derivative, is a white to almost white powder with a molecular weight of 532.47. It is insoluble in water; sparingly soluble in ethanol; and soluble in butanol.
Terconazole - Clinical Pharmacology
Absorption - Following a single intravaginal application of a suppository containing 240 mg 14C-Terconazole to healthy women, approximately 70% (range: 64-76%) of Terconazole remains in the vaginal area during the suppository retention period (16 hours); approximately 10% (range: 5-16%) of the administered radioactivity was absorbed systemically over 7 days. Maximum plasma concentrations of Terconazole occur 5 to 10 hours after intravaginal application of the suppository. Systemic exposure to Terconazole is approximately proportional to the applied dose. The rate and extent of absorption of Terconazole are similar in patients with vulvovaginal candidiasis (pregnant or non-pregnant) and healthy subjects.
Distribution - Terconazole is highly protein bound (94.9%) in human plasma and the degree of binding is independent of drug concentration over the range of 0.01 to 5.0 mcg/mL.
Metabolism - Systemically absorbed Terconazole is extensively metabolized (>95%).
Elimination - Across various studies in healthy women, after single or multiple intravaginal administ...