Terconazole Vaginal Cream
Dosage form: vaginal cream
Drug class:Vaginal anti-infectives
Medically reviewed by Drugs.com. Last updated on Mar 1, 2021.
On This Page
Rx Only
DESCRIPTION
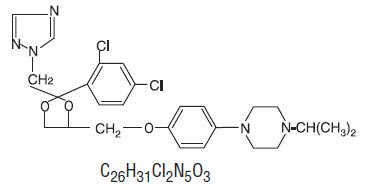
Terconazole Vaginal Cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, compounded in a cream base consisting of butylated hydroxyanisole, cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, stearyl alcohol, and purified water.
The structural formula of terconazole is as follows:
Terconazole, a triazole derivative, is a white to almost white powder with a molecular weight of 532.47. It is insoluble in water; sparingly soluble in ethanol; and soluble in butanol.
CLINICAL PHARMACOLOGY
Absorption
Following a single intravaginal application of a suppository containing 240 mg 14C-terconazole to healthy women, approximately 70% (range: 64 to 76%) of terconazole remains in the vaginal area during the suppository retention period (16 hours); approximately 10% (range: 5 to 16%) of the administered radioactivity was absorbed systemically over 7 days. Maximum plasma concentrations of terconazole occur 5 to 10 hours after intravaginal application of the cream or suppository. Systemic exposure to terconazole is approximately proportional to the applied dose, whether as the cream or suppository. The rate and extent of absorption of terconazole are similar in patients with vulvovaginal candidiasis (pregnant or non-pregnant) and healthy subjects.
Distribution
Terconazole is highly protein bound (94.9%) in human plasma and the degree of binding is independent of drug concentration over the range of 0.01 to 5 mcg/mL.
Metabolism
Systemically absorbed terconazole is extensively metabolized (>95%).
Elimination
Across various studies in ...