Tetracaine
Generic name: Tetracaine hydrochloride
Dosage form: injection
Drug class:Local injectable anesthetics
Medically reviewed by Drugs.com. Last updated on Feb 1, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
Tetracaine HCl Injection, USP
for Prolonged Spinal Anesthesia
Rx only
Description
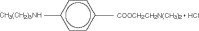
Tetracaine hydrochloride is 2-(Dimethylamino)ethyl p-(butylamino)benzoate monohydrochloride. It is a white crystalline, odorless powder that is readily soluble in water, physiologic saline solution, and dextrose solution. It has the following structural formula:
Tetracaine hydrochloride is a local anesthetic of the ester-linkage type, related to procaine.
1% Solution: A sterile, isotonic, isobaric solution.
Each mL contains:
Active: 10 mg Tetracaine Hydrochloride
Inactives: 7.5 mg Sodium Chloride, Hydrochloric Acid and/or Sodium Hydroxide may be added to adjust pH (3.2 to 6.0) and Water for Injection, USP.
Nitrogen gas has been used to displace the air in the ampules.
This formulation does not contain preservatives.
CLINICAL PHARMACOLOGY
Parenteral administration of Tetracaine hydrochloride stabilizes the neuronal membrane and prevents initiation and transmission of nerve impulses thereby effecting local anesthesia.
The onset of action is rapid, and the duration is prolonged (up to two or three hours or longer of surgical anesthesia).
Tetracaine hydrochloride is detoxified by plasma esterases to aminobenzoic acid and diethylaminoethanol.
INDICATIONS AND USAGE
Tetracaine hydrochloride is indicated for the production of spinal anesthesia for procedures requiring two to three hours.
CONTRAINDICATIONS
Spinal anesthesia with Tetracaine hydrochloride is contraindicated in patients with known h...