Tham
Generic name:tromeThamine
Dosage form: injection, solution
Drug classes:Minerals and electrolytes, Miscellaneous genitourinary tract agents
Medically reviewed by Drugs.com. Last updated on May 23, 2022.
On This Page
For the Prevention and Correction of Severe Metabolic Acidosis
Large Volume Glass Container
Rx only
Tham Description
Tham Solution (tromeThamine injection) is a sterile, non-pyrogenic 0.3 M solution of tromeThamine, adjusted to a pH of approximately 8.6 with glacial acetic acid. It is administered by intravenous injection, by addition to ACD blood for priming cardiac bypass equipment and by injection into the ventricular cavity during cardiac arrest.
Each 100 mL contains tromeThamine 3.6 g (30 mEq) in water for injection. The solution is hypertonic 389 mOsmol/L (calc.). pH 8.6 (8.4-8.7).
The solution contains no bacteriostat, antimicrobial agent or added buffer (except acetic acid for pH adjustment) and is intended only for use as a single-dose injection. When smaller doses are required the unused portion should be discarded.
Tham solution is a parenteral systemic alkalizer and fluid replenisher.
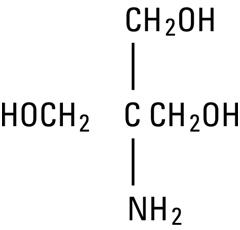
TromeThamine, USP (sometimes called “tris” or “tris buffer”) is chemically designated 2-amino-2-(hydroxymethyl)-1, 3-propanediol, a solid readily soluble in water, also classified as an organic amine buffer. It has the following structural formula:
Water for Injection, USP is chemically designated H20.
Tham - Clinical Pharmacology
When administered intravenously as a 0.3 M solution, tromeThamine act as a proton acceptor and prevents or corrects acidosis by actively binding hydrogen ions (H+). It binds not only cations of fixed or metabolic acids, but also hydrogen ions of carbonic acid, thus increasing bicarbonate anion (HCO3‾). TromeThamine also acts as an osmotic diuretic, increasing urine flow, urinary pH, and excretion of fixed acids, carbon dioxide and electrolytes. A significant fraction of tromeThamine (30% at pH 7.40) is not ionized and th...



