Toposar
Generic name:etoposide
Dosage form: injection, solution, concentrate
Drug class:Mitotic inhibitors
Medically reviewed by Drugs.com. Last updated on Mar 22, 2022.
On This Page
5653
5656
5657
Rx only
Toposar (etoposide injection) should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Severe myelosuppression with resulting infection or bleeding may occur.
Toposar Description
Toposar® (etoposide injection USP) (also commonly known as VP-16) is a semisynthetic derivative of podophyllotoxin used in the treatment of certain neoplastic diseases. It is 4'-demethylepipodophyllotoxin 9-[4,6-0-(R)-ethylidene-β-D-glucopyranoside]. It is very soluble in methanol and chloroform, slightly soluble in ethanol, and sparingly soluble in water and ether. It is made more miscible with water by means of organic solvents.
Toposar (etoposide injection USP) is available for intravenous use as a sterile 20 mg/mL solution in 100 mg (5 mL), 500 mg (25 mL), or 1 g (50 mL) sterile, multiple dose vials. The pH of the clear, yellow liquid is 3.0 to 4.0.
Each mL contains: 20 mg etoposide, USP, 2 mg citric acid anhydrous, 80 mg polysorbate 80, 650 mg polyethylene glycol 300 (57.5% v/v and 65.0% w/v), and 262 mg dehydrated alcohol (33.2% v/v and 26.2% w/v).
The structural formula is:
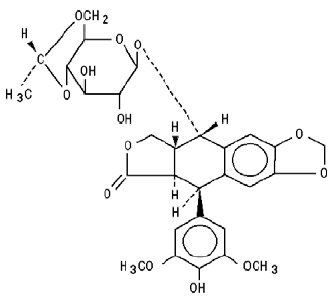
C29H32O13 M.W. 588.56
Toposar - Clinical Pharmacology
Toposar has been shown to cause metaphase arrest in chick fibroblasts. Its main effect, however, appears to be at the G2 portion of the cell cycle in mammalian cells. Two different dose-dependent responses are seen. At high concentrations (10 mcg/mL or more), lysis of cells entering mitosis is observed. At low concentrations (0.3.



