Axid Oral Solution
Generic name:nizatidine
Dosage form: oral solution
Drug class:H2 antagonists
Medically reviewed by Drugs.com. Last updated on Dec 22, 2021.
On This Page
Rx only
The Axid Oral Solution brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Description
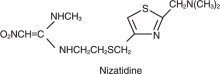
Nizatidine (USP) is a histamine H2-receptor antagonist. Chemically, it is N-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N'-methyl-2-nitro-1,1-ethenediamine.
The structural formula is as follows:
Nizatidine has the empirical formula C12H21N5O2S2 representing a molecular weight of 331.47. It is an off-white to buff crystalline solid that is soluble in water. Nizatidine has a bitter taste and mild sulfur-like odor.
Axid Oral Solution is formulated as a clear, yellow, oral solution with bubble gum flavor and each 1 mL contains 15 mg of nizatidine. Axid Oral Solution also contains the inactive ingredients methylparaben, propylparaben, glycerin, sodium alginate, purified water, sodium chloride, saccharin sodium, sodium citrate dihydrate, citric acid anhydrous, sucrose, bubble gum flavor, artificial sweetness enhancer, and sodium hydroxide.
Clinical Pharmacology in Adults:Nizatidine is a competitive, reversible inhibitor of histamine at the histamine H2-receptors, particularly those in the gastric parietal cells.
Antisecretory Activity—1. Effects on Acid Secretion: Nizatidine significantly inhibited nocturnal gastric acid secretion for up to 12 hours. Nizatidine also significantly inhibited gastric acid secretion stimulated by food, caffeine, betazole, and pentagastrin (Table 1).