Trivora
Generic name:levonorgestrel and ethinyl estradiol
Dosage form: tablet
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on Mar 1, 2022.
PHYSICIAN LABELING
Rx only
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
On This Page
Trivora Description
Each Trivora cycle of 28 tablets consists of three different drug phases as follows: Phase 1 comprised of 6 blue tablets, each containing 0.050 mg of levonorgestrel (d(-)13 beta-ethyl-17-alpha-ethinyl-17- beta-hydroxygon-4-en-3-one), a totally synthetic progestogen, and 0.030 mg of ethinyl estradiol (19- nor-17α-pregna-1,3,5(10)-trien-20-yne-3, 17-diol); phase 2 comprised of 5 white tablets, each containing 0.075 mg levonorgestrel and 0.040 mg ethinyl estradiol; and phase 3 comprised of 10 pink tablets, each containing 0.125 mg levonorgestrel and 0.030 mg ethinyl estradiol; then followed by 7 peach inert tablets. The inactive ingredients present in the blue, white and pink tablets are lactose monohydrate, magnesium stearate, povidone and starch (corn). Each blue tablet also contains FD&C Blue #1. Each pink tablet also contains FD&C Red #40. Each inactive peach tablet contains the following inactive ingredients: anhydrous lactose, FD&C Yellow #6, lactose monohydrate, magnesium stearate and microcrystalline cellulose.
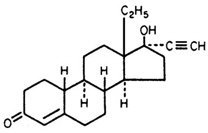 | 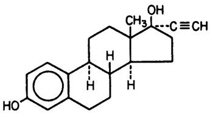 | |
| Levonorgestrel | Ethinyl Estradiol |



