Twinject Auto-Injector
Generic name:epinephrine
Dosage form: injection
Drug classes:Adrenergic bronchodilators, Catecholamines, Vasopressors
Medically reviewed by Drugs.com. Last updated on May 23, 2022.
On This Page
Available as: 0.3 mg 0.15 mg
each dose delivers 0.15 mg or 0.3 mg of epinephrine
PRESCRIBING INFORMATION
The Twinject brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Twinject Auto-Injector Description
Twinject Auto-Injector contains 1.1 mL epinephrine injection, USP 1:1000 (1 mg/mL), from which two doses of either 0.15 mg (0.15 mL) or 0.3 mg (0.3 mL) each are available for use by injection. The first dose is administered by auto-injection after the patient prepares and fires Twinject as directed. A second dose can be manually administered following a partial disassembly of Twinject. The remaining volume is not available for use and should be discarded. See PATIENT DIRECTIONS FOR USE on the accompanying Patient Information Leaflet.
Each dose of epinephrine injection, USP 1:1000 contains either 0.15 mg or 0.3 mg l-epinephrine, sodium chloride, chlorobutanol and sodium bisulfite, all sealed under nitrogen.
Epinephrine is a sympathomimetic catecholamine. Its naturally occurring l-isomer, which is twenty times as active as the d-isomer, is obtained in pure form by separation from the synthetically produced racemate.
Chemically, epinephrine is 1-(3,4-dihydroxyphenyl)-2-(methylamino)ethanol with the following structure:
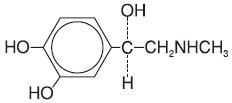
Epinephrine deteriorates rapidly on exposure to air or light, turning pink from oxidation to adrenochrome and brown from the formation of melanin. Epinephrine solutions that show evidence of discoloration should be discarded.
Twinject contains no latex.
Twinject Auto-Injector - Clinical Pharmacology
Epinephri...



