Uramaxin Foam
Generic name:urea
Dosage form: foam
Drug class:Topical emollients
Medically reviewed by Drugs.com. Last updated on Jul 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Rx Only
In a vehicle containing Ammonium Lactate
For external use only. Not for ophthalmic use.
Uramaxin Foam Description
Uramaxin™ (20% Urea) Foam is a keratolytic emollient moisturizer. Each gram contains 20% urea, purified water, ammonium lactate, white petrolatum, octyl palmitate, caprylic/capric triglyceride, hydrogenated polyisobutene, propylene glycol, rice starch, polysorbate 60, cyclomethicone, glyceryl stearate & PEG-100 stearate, cetearyl alcohol & cetearyl glucoside, polysorbate-20, phenoxyethanol, cetyl alcohol, dimethicone, potassium sorbate, allantoin, tocopheryl acetate, xanthan gum. In propellants isobutane & propane & butane.
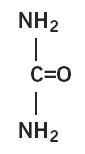
Urea is a diamide of carbonic acid with the following chemical structure:
Uramaxin Foam - Clinical Pharmacology
Urea gently lyses/dissolves the intercellular matrix of surface skin cells loosening and allowing a shedding of rough, thickened and scaly hyperkeratotic skin. Urea also moisturizes and softens skin.
PHARMACOKINETICS
The mechanism of action of topically applied Urea is not yet known.
INDICATIONS AND USES
For softening, smoothing and removing rough scaling hyperkeratotic skin in conditions such as xerosis, ichthyosis, skin cracks and fissures, dermatitis, eczema, psoriasis, keratoses and calluses.
Contraindications
Known hypersensitivity to any of the listed ingredients. Discontinue if hypersensitivity is observed. Sun exposure to areas of skin treated wi...



