Uramaxin Pads
Generic name:urea
Dosage form: topical cloth
Drug class:Topical emollients
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
For external use only. Not for ophthalmic use.
On This Page
DESCRIPTION: Uramaxin® (45% Urea) Pads contain a 45% solution of urea on a textured cloth. Each gram of medicated solution contains 45% urea, camphor, edetate disodium, eucalyptus oil, hydroxyethyl cellulose, menthol, propylene glycol and purified water.
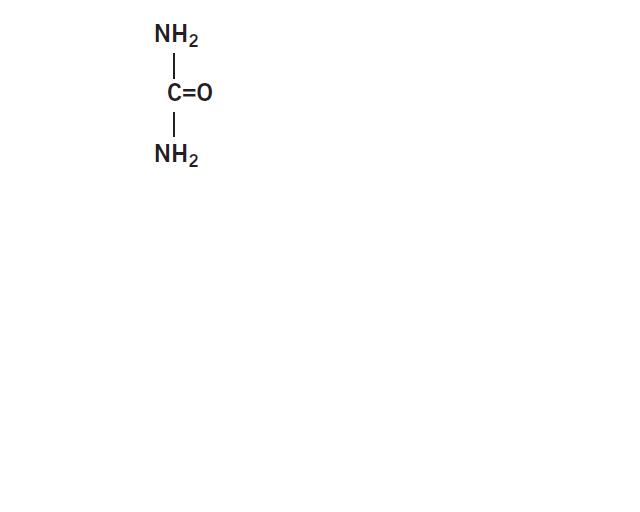
Urea is a diamide of carbonic acid with the following chemical structure:
CLINICAL PHARMACOLOGY: Urea gently dissolves the intercellular matrix, which results in loosening of the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas.
PHARMACOKINETICS: The mechanism of action of topically applied Urea is not yet known.
INDICATIONS AND USES: For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis pilaris, keratosis palmaris, keratoderma, corns and calluses.
CONTRAINDICATIONS: Known hypersensitivity to any of the listed ingredients.
WARNINGS: For external use only. Avoid contact with eyes, lips or mucous membranes.
PRECAUTIONS: This medication is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use.
PREGNANCY: Pregnancy Category B. Animal reproduction studies have revealed no evidence of harm to the fetus,..



