Urevaz Cream
Generic name:urea
Dosage form: cream
Drug class:Topical emollients
Medically reviewed by Drugs.com. Last updated on Apr 21, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
UREVAZ
Urea Cream 44%
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
Rx Only
Urevaz Cream Description
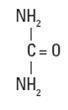
Urea, 44 % is a keratolytic emollient which is gentle, yet potent, tissue softener for nails and/or skin. Each gram of Urea Cream, 44 % contains 44% urea, edetate disodium dihydrate, glycerin, hydroxethylcellulose, PEG-6 caprylic/capric glyceride, purified water,phenoxyethanol,ethylexylglycerin and xanthan gum. Urea is a diamide of carbonic acid with the following chemical structure:
Urevaz Cream - Clinical Pharmacology
Urea gently dissolves the intercellular matrix which results in loosening the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas. Urea also hydrates and gently dissolves the intercellular matrix of the nail plate, which can result in the softening and eventual debridement of the nail plate.
Pharmacokinetics
The mechanism of action of topically applied urea is not yet known.
INDICATIONS AND USES
For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or prurient debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis, keratoderma, corns and calluses, as well as damaged, ingrown and devitalized nails.



