Utopic Cream
Generic name:urea
Dosage form: cream
Drug class:Topical emollients
Medically reviewed by Drugs.com. Last updated on Nov 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
DESCRIPTION:
Each gram contains 410 mg of urea in a vehicle consisting of: water, Carbomer Ultrez 10, xanthan gum, propylene glycol, cetyl alcohol, mineral oil, petrolatum, glyceryl stearate, dimethyl isosorbide and sodium hydroxide.
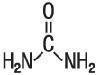
Urea is a diamide of carbonic acid with the following chemical structure:
CLINICAL PHARMACOLOGY:
Urea gently dissolves the intercellular matrix which results in loosening the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas of the skin.
Pharmacokinetics: The mechanism of action of topically applied urea is not yet known.
INDICATIONS:
This product is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, xerosis, ichthyosis, skin cracks and fissures, dermatitis, eczema, psoriasis, keratoses and calluses.
CONTRAINDICATIONS:
This product is contraindicated in persons with known or suspected hypersensitivity to any of the ingredients of the product.
WARNINGS:
KEEP OUT OF REACH OF CHILDREN.
PRECAUTIONS:
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
General: This product is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use and consult a physician.
Information for Patients: Patients should discontinue the use of this product if the condition becomes worse or if a rash dev...



